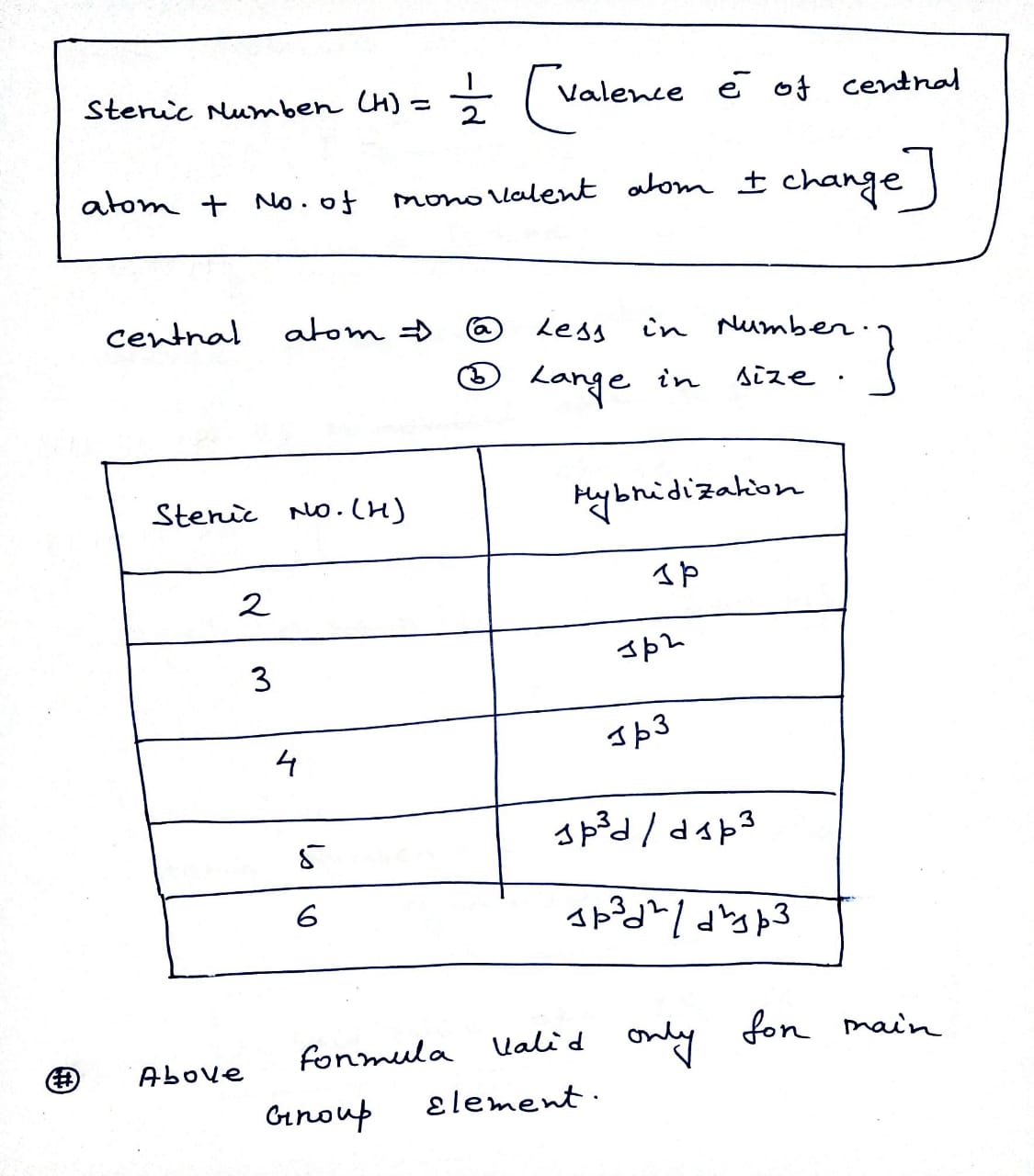
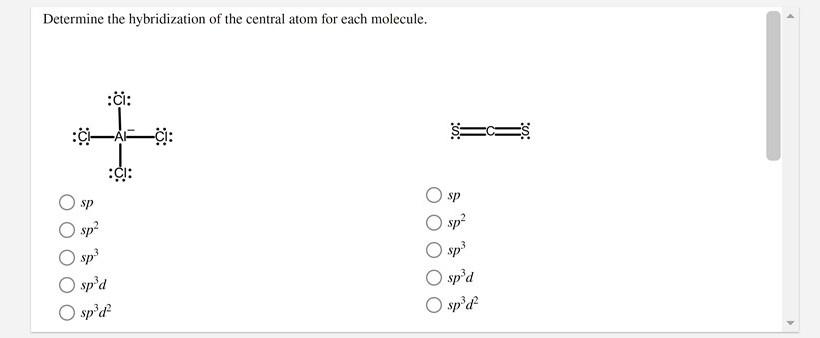
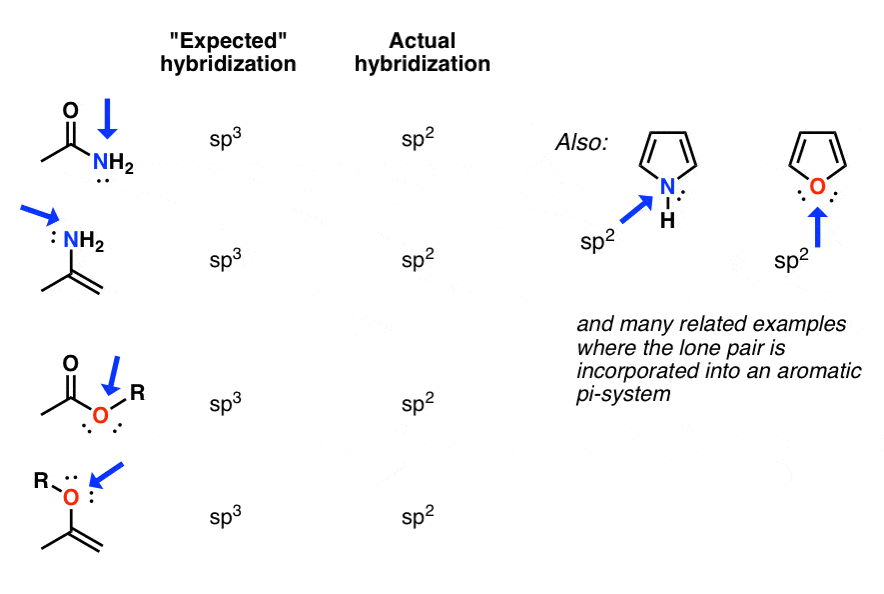
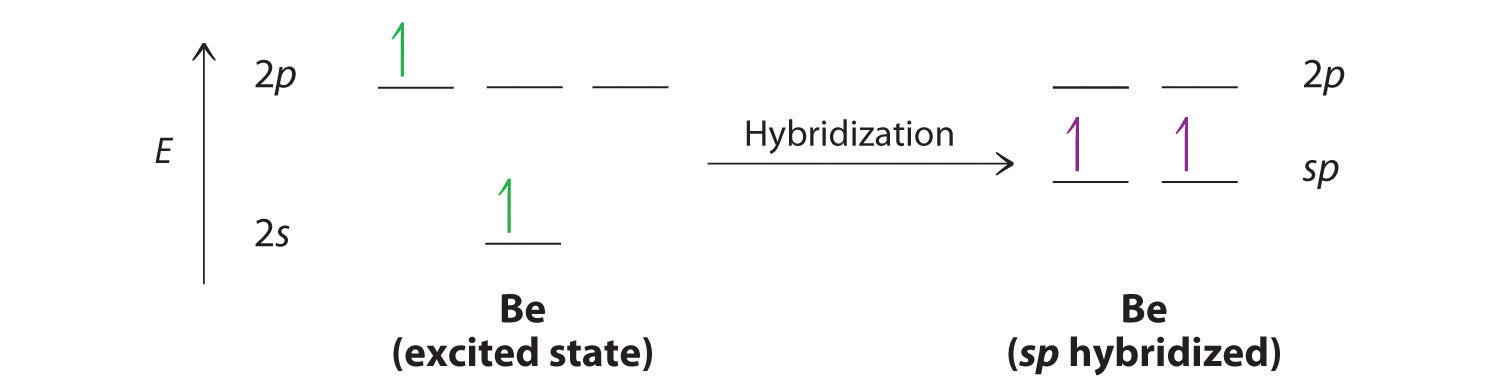
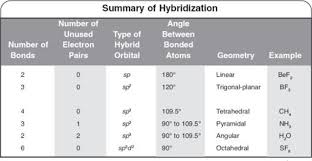
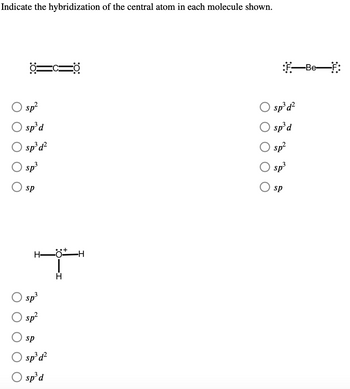
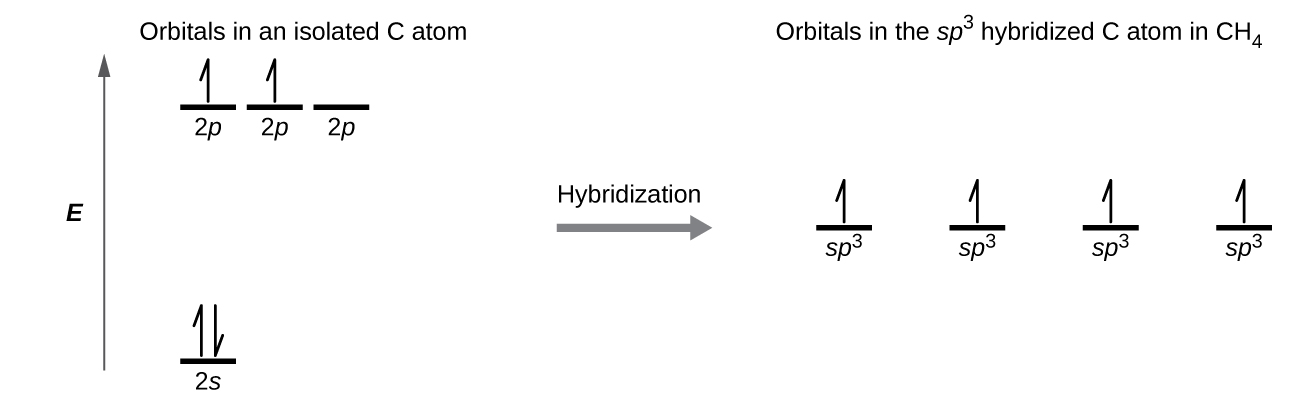
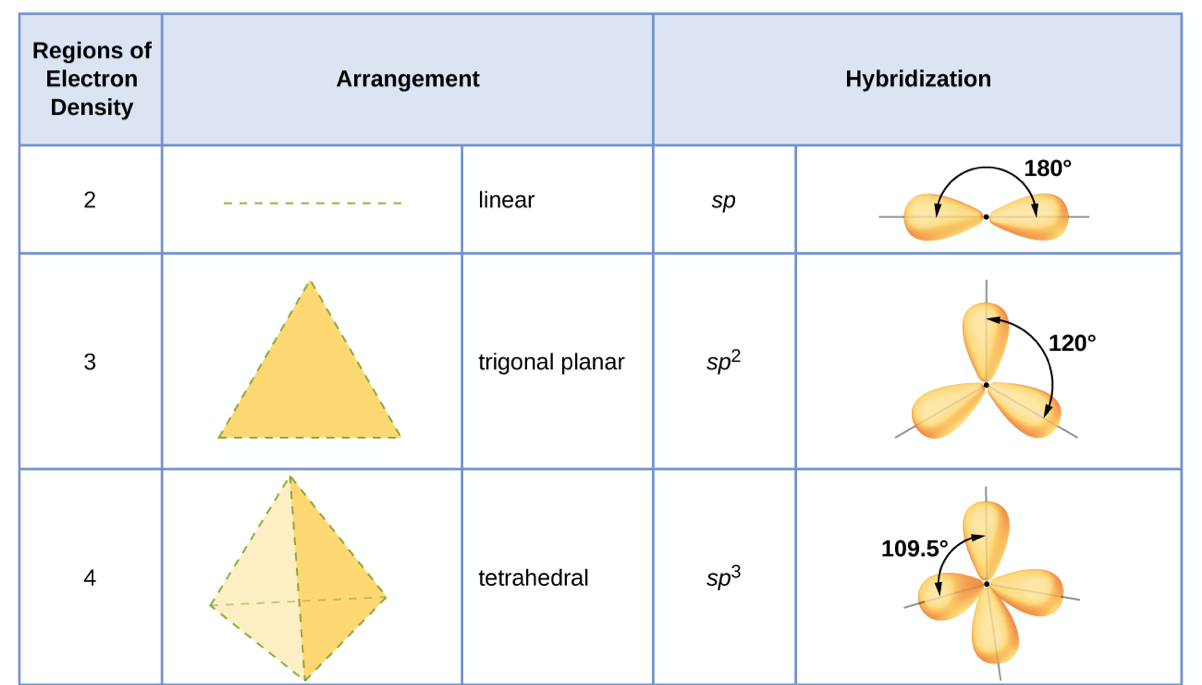
Hybridization of central atoms in the moleculesN(CH3)3 and N(SIH3) respectively are(1) sp2 and sp2(2) sp3 and sp3(3) sp2 and sp34) sp3 and sp2
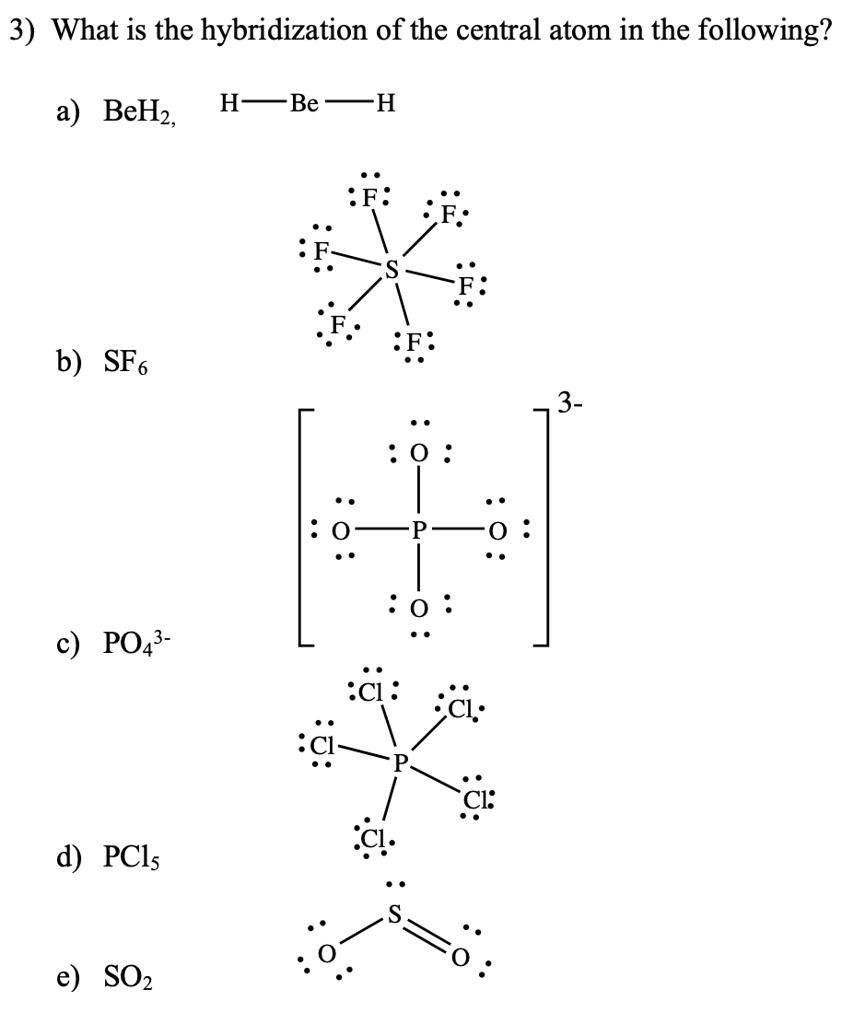
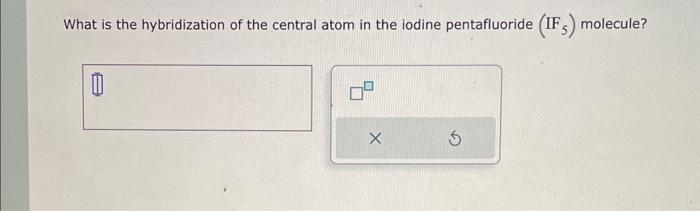
SOLVED: What is the hybridization of the central atom in the following? H2O a) BeH2, b) SF6 c) PO3^- d) PCl5 e) SO2
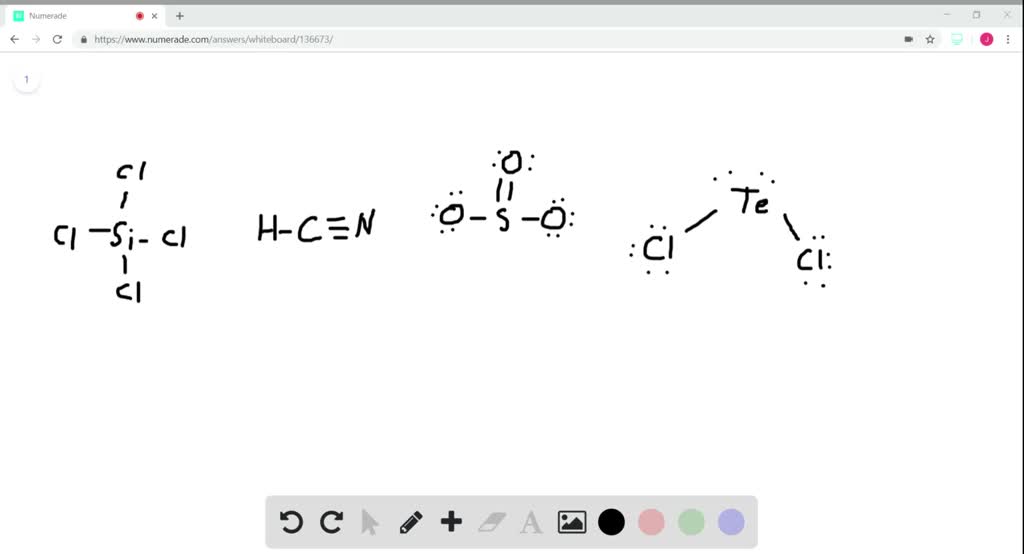
19. In which one of the following species the central atom has the type of hybridization which is not the same as that present in other three? 1)SF4 2)I3 3)SbCl3 4)PCl5

In which of the following pairs of molecules/ions, the central atoms have sp^{2} hybridization?BF_{3} and NO{_{2}}^{-}NO{_{2}}^{-} and NH_{3}BF_{3} and NH{_{2}}^{-}NH{_{2}}^{-} and H_{2}O