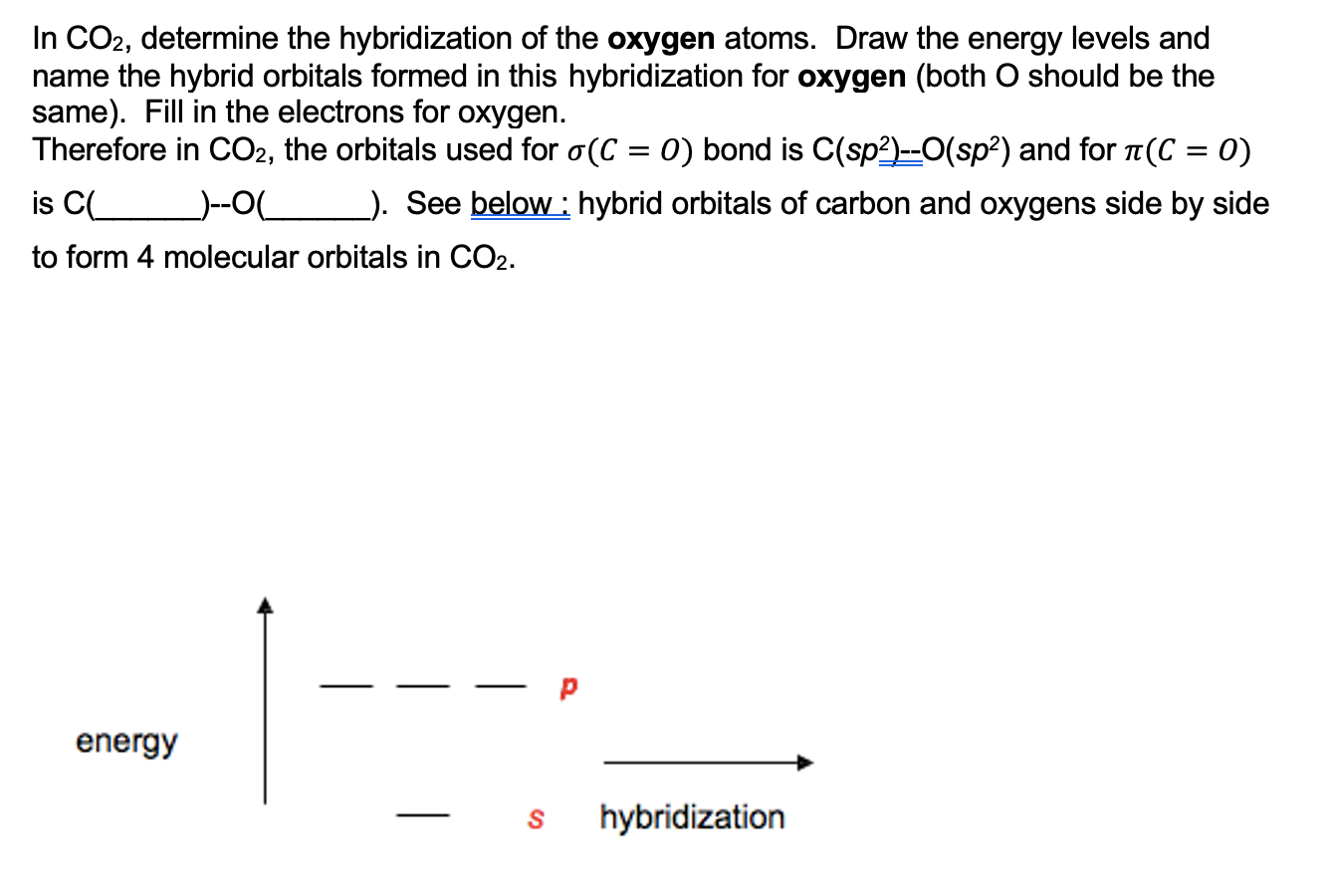
Draw a Lewis structure and an orbital picture for carbon dioxide, CO2. What kind of hybridization does the carbon atom have? What is the relationship between CO2 and allene? | Homework.Study.com

The molecule CO2 has two C-O double bonds. Describe the bonding in the CO2 molecule. Which involves hybrid - brainly.com

p–d Orbital Hybridization Induced by p-Block Metal-Doped Cu Promotes the Formation of C2+ Products in Ampere-Level CO2 Electroreduction | Journal of the American Chemical Society
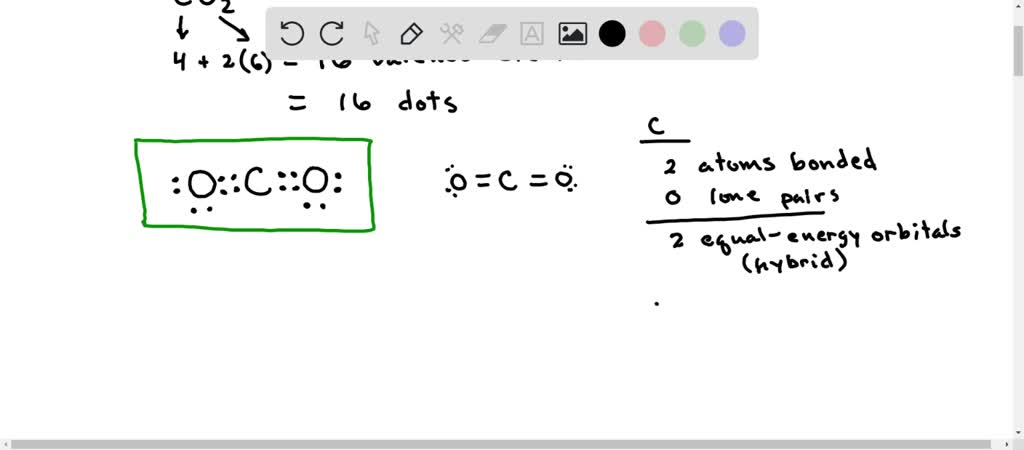
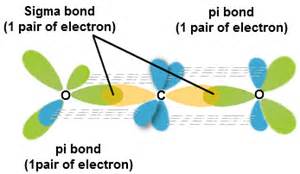
SOLVED: Draw a Lewis structure for CO2. Determine the hybridization on the carbon atom. Which orbitals of the carbon atom remain remain unhybridized? How many sigma and pi bonds are formed?

p–d Orbital Hybridization Induced by p-Block Metal-Doped Cu Promotes the Formation of C2+ Products in Ampere-Level CO2 Electroreduction | Journal of the American Chemical Society

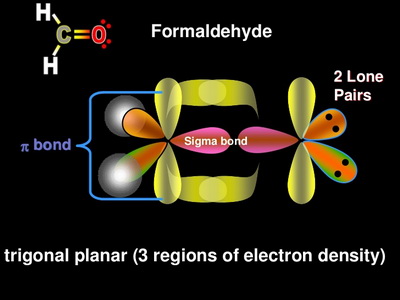
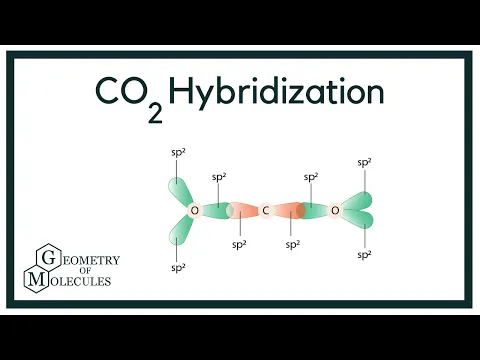
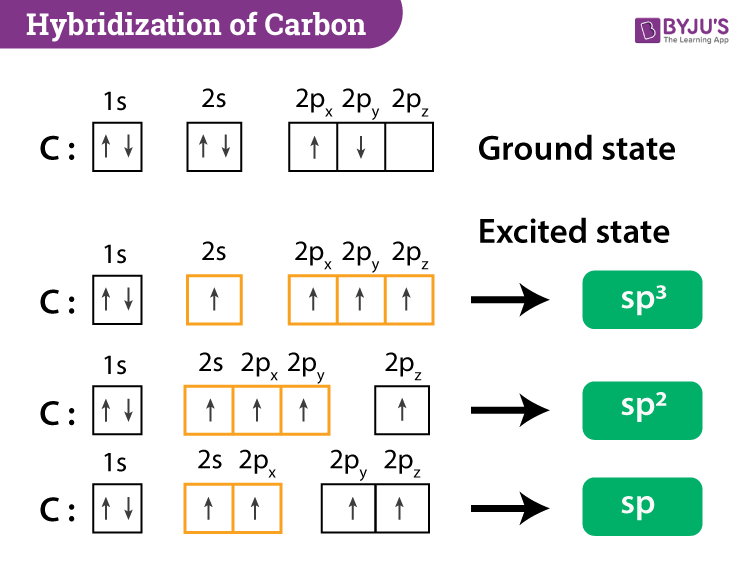
![SOLVED] In CO2 hybridization of carbon is sp sp2 sp3 None of t - Self Study 365 SOLVED] In CO2 hybridization of carbon is sp sp2 sp3 None of t - Self Study 365](https://static.tllms.com/moodle-migration/47309_7fc775895c5b6da80cbe1e11e0d869d746d07138_S-1.PNG)