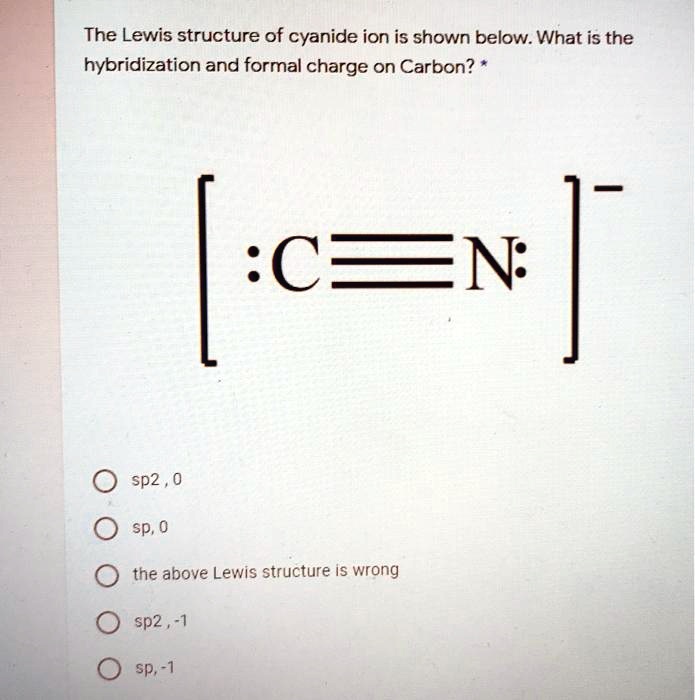
SOLVED: The Lewis structure of cyanide ion is shown below. What is the hybridization and formal charge on Carbon? :C=N 0 sp2 , 0 sp; 0 the above Lewis structure iS wrong
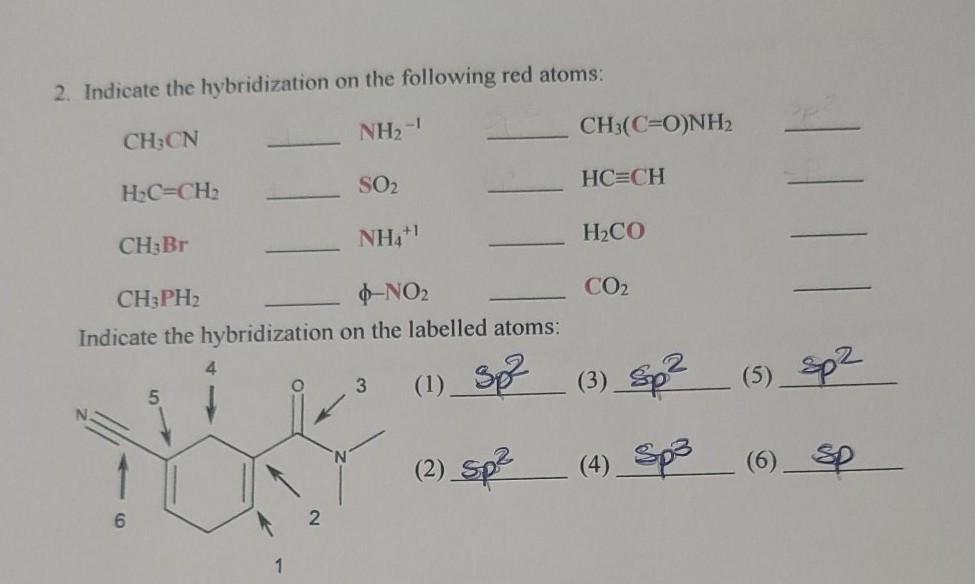
How to find the hybridizationof[Ni(CN)4]2 n ntand the d orbital used in it. What is the hybridization of NO2?
![For the complex [Fe(CN)6]3–, write the hybridization type, magnetic character and spin nature of the complex. - Chemistry | Shaalaa.com For the complex [Fe(CN)6]3–, write the hybridization type, magnetic character and spin nature of the complex. - Chemistry | Shaalaa.com](https://www.shaalaa.com/images/_4:cd81081959db46f9b02647463e8defe4.png)
For the complex [Fe(CN)6]3–, write the hybridization type, magnetic character and spin nature of the complex. - Chemistry | Shaalaa.com

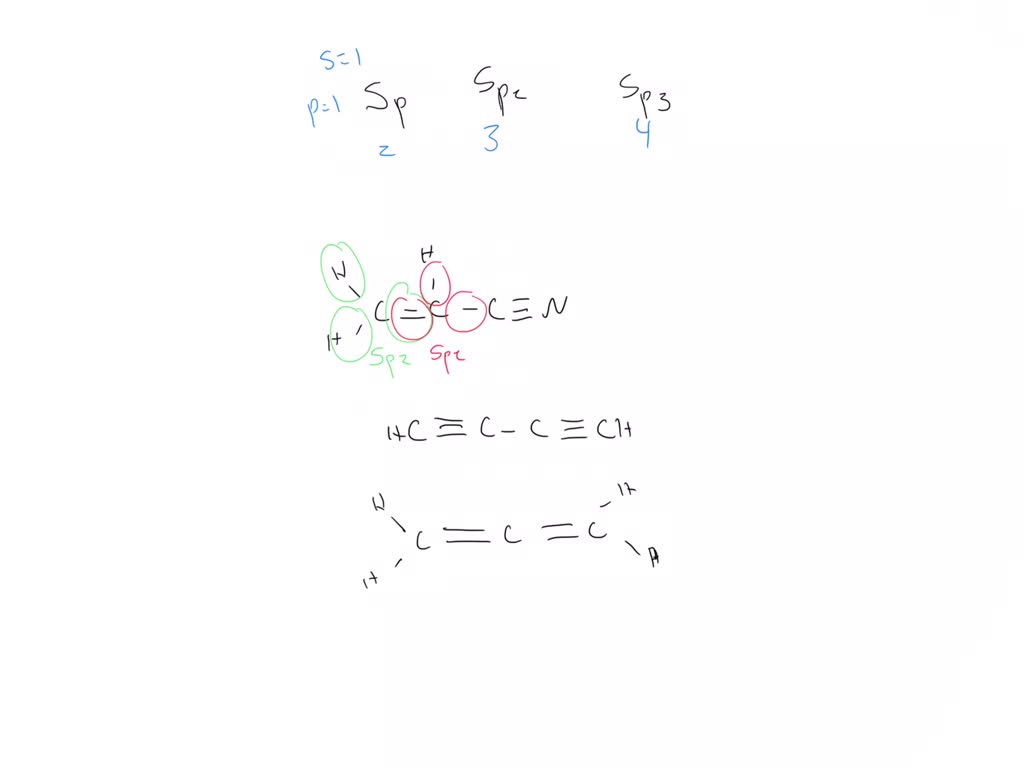
Carbon atoms in C_2(CN)_4 are:sp hybridizedsp^2 hybridizedsp and sp^2 hybridizedsp, sp^2 and sp^3 hybridized
The hybridization and magnetic nature of [Mn(CN)6]^4- and [Fe(CN)6]^3–, respectively are: - Sarthaks eConnect | Largest Online Education Community
![a) For the complex { left[ Fe{ left( CN right) }_{ 6 } right] }^{ 3- }, write the hybridization type, magnetic character and spin nature of the complex. (Atomic number : a) For the complex { left[ Fe{ left( CN right) }_{ 6 } right] }^{ 3- }, write the hybridization type, magnetic character and spin nature of the complex. (Atomic number :](https://search-static.byjusweb.com/question-images/toppr_ext/questions/994279_471862_ans_274df12034c14a6ea02085d9c811233c.bmp)
a) For the complex { left[ Fe{ left( CN right) }_{ 6 } right] }^{ 3- }, write the hybridization type, magnetic character and spin nature of the complex. (Atomic number :


![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-1.png)
![The hybridization involved in complex [Ni(CN)(4)]^(2-) is (At. No . Ni The hybridization involved in complex [Ni(CN)(4)]^(2-) is (At. No . Ni](https://static.doubtnut.com/ss/web-overlay-thumb/1942248.webp)
![Write the hybridization and shape of the following complexe : [Ni(CN)4]2– - Chemistry | Shaalaa.com Write the hybridization and shape of the following complexe : [Ni(CN)4]2– - Chemistry | Shaalaa.com](https://www.shaalaa.com/images/_4:ffa0132b7d904ae4be8cc8b622fc0250.png)

![SQP] Using Valence bond theory, explain the following in relation to SQP] Using Valence bond theory, explain the following in relation to](https://d1avenlh0i1xmr.cloudfront.net/6c2d1cd5-056b-42bc-8a63-e60054c8cb37/question-27---using-valence-bond-theory---teachoo.jpg)
![Find the hybridization of [Cr(CN)6]-3 using VBT ? - Brainly.in Find the hybridization of [Cr(CN)6]-3 using VBT ? - Brainly.in](https://hi-static.z-dn.net/files/dad/31ca5894179e2d58f3aafeb5035856c8.png)
![The hybridization of `Fe` in `K_4[Fe(CN)_6]` complex is: - YouTube The hybridization of `Fe` in `K_4[Fe(CN)_6]` complex is: - YouTube](https://i.ytimg.com/vi/2E532r8xHTg/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGHIgQyg4MA8=&rs=AOn4CLAYoqZSx8xdl9GxLDiVnG4D38Kb_w)
![What is the hybridization for [NiCN4]2 ? What is the hybridization for [NiCN4]2 ?](https://byjus-answer-creation.s3.amazonaws.com/uploads/2.14.jpg_img_upload_solution_2022-05-30%2005:07:29.453226.png)